Day :
Keynote Forum
MarÃa Ramos Payan
Instituto de Microelectrónica de Barcelona IMB-CNM (CSIC), Spain
Keynote: Double-flow or stopped-flow conditions as different operating working modes for microfluidic devices in sample preparation depending of the application field
Time : 0

Biography:
Abstract:
Keynote Forum
Mohamed Ibrahim BADAOUI
Batna-1 University
Keynote: Chemical composition of medicinal plant of Atractylis
Time : 0

Biography:
Abstract:
Keynote Forum
Manar Obada
Menoufia University
Keynote: Chromatographic Detection of Inborn Error of Metabolism in Egyptian Pediatrics, Two years experiences
Time : 0

Biography:
Abstract:
- High Performance Liquid Chromatography | Applications of Chromatography |Novel Techniques in Chromatography
Location: Olimpica 2

Chair
Dusan Berek
Polymer Institute - SAS, Slovakia

Co-Chair
Jorge Costa Pereira
University of Coimbra, Portugal
Session Introduction
Ng Mei Han
Malaysian Palm Oil Board, Malaysia
Title: Chromatographic analyses of tocols in palm in the absence of authentic standards
Time : 11:20-11:50

Biography:
Abstract:
Publications
- Goh E P S, Ng M H, Choo Y M, Nasrulhaq Boyce A and Chuah C H (2016) Production of tocols nanoemulsion by ultrasonication. J. Oil Palm Res., 28:121-130.
- Ng M H and Choo Y M (2015) Packed supercritical fluid chromatography for the analyses and preparative separations of palm oil minor components. American Journal of Analytical Chemistry 6(8).
- Goh P S, Ng M H, Choo Y M, Amru N B and Chuah C H (2015) Production of nano-emulsions from palm-based tocotrienol rich fraction by micro-fluidization. Molecules 20.
- Ng M H and Choo Y M (2013) Isolation and recovery of phytonutrients in palm by isocratic and isobaric flash chromatography. J. Oil Palm. Res. 25(2):165–169.
- Ng M H and Choo Y M (2011) Chromatographic analyses of tocopherols and tocotrienols in palm oil. J. Chrom. Sci. 50(3):283-286.
Dina Shokry
University of Huddersfield, UK
Title: Bile salt: A biosurfactant or a pharmacokinetic predictive tool
Time : 11:50-12:20

Biography:
Dina Shokry completed her Bachelor degree in Pharmacy in 2009 at Ain Shams University then Master’s degree in Analytical Chemistry at Cairo University in 2013. Now, she is about to complete her PhD as a member of Dr Waters group for fi nding alternatives to animal testing at Huddersfi eld University. She worked as a Teaching Assistant then as an Assistant Lecturer of Analytical Chemistry at Future University. She produced high quality research that was published in anumber of reputed peer reviewed journals and presented her work in nine conferences. Her work is focused on developing models for prediction of human intestinal absorption through in vitro-in vivo correlation studies which has economic impact in the pharmaceutical industry fi eld. She developed prediction models from MLC,solubilization and permeation studies where the obtained in vitro data correlated well with the in vivo absorption data and resulted in two recently published papers.
Abstract:
Most of the new released drug compounds are formulated as orally administered drugs due to the convenience of the oral administration route. However, the properties of some compounds could be incompatible with oral administration. In fact, major fi nancial losses were suff ered by pharmaceutical industry because some new drugs were discovered to have poor bioavailability aft er their oral administration when tested in later clinical stage of development. Th erefore, drugs with poor aqueous solubility and oral bioavailability that are considered poor candidates should be spotted as soon as possible before reaching fi nal clinical stages of development where the costs spent on research carried out in such stages for studying the biopharmaceutical properties of the drug is signifi cantly high, it is even better to discover these properties before the drug is synthesized to save time and money. Over the past three decades, there has been growing interest in the prediction of the biopharmaceutical properties as aqueous solubility and intestinal permeability of new drug entities (NDE) that resulted in the development of a large number of experimental (in vitro and in situ) and mathematical models. In addition to being cost eff ective and time saving, some of these models help in the determination of best drug candidates during drug discovery and development stage. Drug intestinal permeability is one of the most important biopharmaceutical properties that are worth investigating and predicting using the previously mentioned models. In the spectroscopic and permeation methods, we developed mathematical models generated for prediction of human intestinal absorption (HIA) through the determination of the micelle/water partition coeffi cients (logKxm/a) for a series of 20 compounds using UV spectroscopy and also through determination of the permeation constants (log Kp) of a number of drugs through gels made from bile salt saturated with infinite dose of these drugs. Prediction models with good predictability were developed using the obtained data from both methods along with the reference absorption data and other physicochemical properties to develop prediction equations through simple and multiple linear regression respectively. In another work, we developed a model using MLC method which was proved successful for prediction of HIA.
Mellissa Graewert
EMBL Hamburg Outstation, Germany
Title: Synchrotron radiation and size exclusion chromatography
Time : 12:20-12:50

Biography:
Melissa Graewert is a Structural Biologist. Currently, she works at EMBL's Outstation in Hamburg, Germany located at PETRA III, one of the most brilliant storagering-based X-ray radiation sources in the world. Her expertise includes “Biophysical and structural characterization of proteins”. Her main research focus is on “The implementation and constant development of small angle X-ray scattering as an emerging technique for the characterization of biological therapeutics such as monoclonal antibodies”.
Abstract:
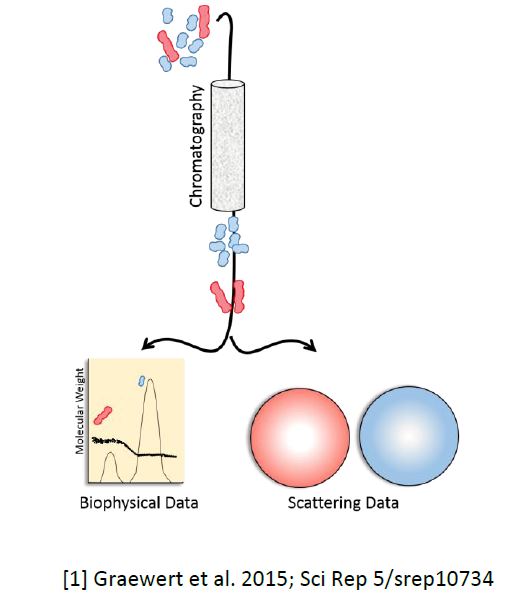
Small angle X-ray scattering (SAXS) is a universal and powerful method to analyze proteins and other macromolecules in solution, in a broad range of sizes and conditions. Th e synergistic improvement in hardware as well as soft ware over the last decade has transformed SAXS into a high-through put technique, which became highly attractive for the pharmaceutical industry. SAXS provides direct insights in the quaternary state; however, it is often hampered by inherent sample polydispersity. At EMBL’s P12 beamline (@Petra III, DESY, Hamburg, Germany), we are developing novel in-line purifi cation systems such as the implementation of an extended size exclusion chromatography set-up for the parallel biophysical and SAXS characterization on separated components. Th e eluting protein is suffi ciently concentrated and pure, so that SAXS data can be directly collected and used for structural and biophysical studies. Modes of access to this set-up (including European funded translational activities such as iNEXT and industrial service provision) are discussed.
Aleš Štrancar
BIA Separations, Slovenia
Title: High resolution monolithic columns - enabling tool for understanding viral structures and their purity
Time : 13:50-14:20

Biography:
Abstract:

Giacomo Russo
Università degli Studi di Napoli Federico II, Italy
Title: Prediction and mechanism elucidation of analyte retention on phospholipid stationary phases (IAM-HPLC) by in silico calculated physico-chemical descriptors
Time : 14:20-14:50

Biography:
Giacomo Russo is a Post-doctoral Scientist in Pharmaceutical Sciences. His research fi eld is intended to elucidate the mechanisms of drug interactions with biological membranes involved in bioavailability and distribution processes. His additional interest is in the development and validation of analytical methods aimed at determining endocrine disrupting agents in food/beverage and biological matrices.
Abstract:
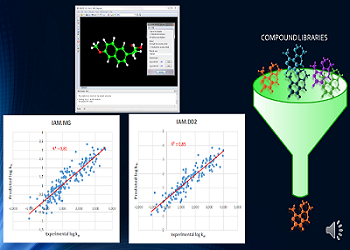
The present study proposes a method for an in silico calculation of phospholipophilicity. Phospholipophilicity is intended as the measure of analyte affi nity for phospholipids; it is currently assessed by HPLC measures of analyte retention on phosphatidylcholine-like stationary phases (IAM-Immobilized Artifi cial Membrane) resulting in log kWIAM values. Due to the amphipathic and electrically charged nature of phospholipids, retention on these stationary phases results from complex mechanisms, being aff ected not only by lipophilicity (as measured by n-octanol/aqueous phase partition coeffi cients, log P) but also by the occurrence of polar and/or electrostatic intermolecular interaction forces. Diff erently from log P, to date no method has been proposed for in silico calculation of log kWIAM. Th e study is aimed both at shedding new light into the retention mechanism on IAM stationary phases and at off ering a high-throughput method to achieve such values. A wide set of physicochemical and topological properties were taken into account, yielding a robust fi nal model including four in silico calculated parameters (lipophilicity, hydrophilic/lipophilic balance, molecular size, and molecule fl exibility). The presented model was based on the analysis of 205 experimentally determined values, taken from the literature and measured by a single research group to minimize the inter laboratory variability; such model is able to predict phospholipophilicity values on both the two IAM stationary phases to date marketed, i.e., IAM.PC.MG and IAM.PC.DD2, with a fairly good degree (r2=0.85) of accuracy. The present work allowed the development of a free on-line service aimed at calculating log kWIAM values of any molecule included in the PubChem database, which is freely available at http://nova.disfarm.unimi.it/logkwiam.html
Publications
- L Grumetto, G Russo and F Barbato (2016) Polar interactions drug/phospholipids estimated by IAM-HPLC vs. cultured cell line passage data: their relationships and comparison of their effectiveness in predicting drug human intestinal absorption. Int. J. Pharm., 500 (2016):275–290.
- L Grumetto, G Russo and F Barbato (2016) Immobilized artificial membrane HPLC derived parameters vs. PAMPA-BBB data in estimating in situ measured blood–brain barrier permeation of drugs. Mol. Pharm.
- L Grumetto, G Russo and F Barbato (2015) Relationships between human intestinal absorption and polar interactions drug/phospholipids estimated by IAM-HPLC. Int. J. Pharm. 489 (2015): 186–194.
- L Grumetto, G Russo and Barbato (2014) Indexes of polar interactions between ionizable drugs and membrane phospholipids measured by IAM-HPLC: their relationships with data of blood-brain barrier passage. Eur. J. Pharm. Sci., 65(2014):139–146.
Yadira S Prieto Curbelo
Center for Molecular Immunology, Cuba
Title: Development and validation of analytical methods based on RP‑HPLC: Quantifying HER1 extracellular domain in culture supernatant and peptide mapping of a monoclonal antibody
Time : 14:50-15:20

Biography:
Abstract:
- Prieto Y, García K and Ochoa D (2016) Development and validation of a method for quantifying HER1 extracellular domain in culture supernatant by RP-HPLC. Chromatographia 79:311–318.
- Garcia K, Prieto Y, Raymond J, Rabasa E, Sánchez B, de la Luz K and Castillo A (2015) Assessment of the impact of manufacturing changes on the physicochemical properties and biological activity of Her1-ECD vaccine during product development. Vaccine 33:4292–4299.
- Prieto Y, Rojas L, Hinojosa L, González I, Aguiar D, de la Luz K, Castillo A and Pérez R (2011) Towards the molecular characterization of the stable producer phenotype of recombinant antibody-producing NS0 myeloma cells. Cytotechnology 63:351–362..
- 4. León D, Prieto Y, Fernández E, Pérez N, Montero J, Palacios J, Bultéd D, de la Luz K, Peña V, Ferro W, Sánchez B, Valdés R and Castillo A (2009) Purification process development for HER1 extracellular domain as a potential therapeutic vaccine. Journal of Chromatography B, 877:3105–3110.
Hermes Licea Perez
GlaxoSmithKline, USA
Title: Applications of supercritical fluid chromatography for chiral metabolite separations in drug metabolism and pharmacokinetics environment
Time : 15:35-16:05

Biography:
Hermes Licea Perez is a Senior Scientifi c Advisor and Technology Leader in Department of Bioanalysis, Immunogenicity & Biomarkers at GlaxoSmithKline, USA. He has been recently selected as GSK Fellow for his scientifi c contribution to the analytical community at GSK. He has completed his Master of Science degree in Chemistry at Moscow State University and a PhD degree at Stockholm University. His PhD research was focused on “Quantifi cation of haemoglobin adducts of industrial chemicals under the supervision of Prof. Siv Osterman-Golkar”. His interests at GlaxoSmithKline include method development and validation of pharmaceutical drugs and metabolites in biological matrices using techniques such as protein precipitation, Solid Phase Extraction (SPE), Liquid Liquid Extraction (LLE), and chemical derivatization (chiral and achiral) for LC (or SFC)-MS/MS detection.
Abstract:
In recent years, interest has expanded to perform chiral separations by supercritical fl uid chromatography (SFC) which is proven to be superior to conventional liquid chromatography in separating structurally related compounds, such as diastereoisomers and enantiomers. Several examples will be described for separation of multiple stereoisomers in biological samples, confi rming SFC to be a powerful tool for stereoisomeric resolution for drug metabolism and pharmacokinetics (DMPK) applications. Two of these examples are summarized below: Gradient UPLC methodologies have previously been applied to separate a drug development compound and its six polyoxygenated metabolites (M2-M6 and M13), supporting numerous non-clinical and clinical PK studies. However, each of these metabolites exists in diff erent stereoisomeric forms, resulting in 14 separate species. Initial attempts at developing UPLC methodologies were not capable of adequately separating these complex species; separation was unsuccessful using chemical derivatization, chiral and conventional reversed-phase liquid chromatography. Th e application of SFC is described herein to separate this complex mixture of 14 stereoisomeric metabolites; these data provided important data on which species circulate in human. SFC in combination with chemical derivatization was proven superior for separation of four diastereomeric species of another drug development compound.Th is method was fully validated and applied to evaluate potential in vivo chiral conversion in pooled clinical and preclinical samples.
Taghreed Alsufyani
Taif University, KSA
Title: Waterborne metabolites as indicators to the growth phases of macroalga Ulva (Chlorophyta) in aquacultures
Time : 16:05-16:35

Biography:
Taghreed Alsufyani is an Assistance Professor of Bioorganic Chemistry at Taif University. She received her Bachelor and Master degrees at King Abdul Aziz University, Jeddah, KSA. After that, she joined Chemistry department at Taif University where she got a scholarship to join PhD program at Friedrich Schiller University Jena, Germany, under the supervision of Dr. Thomas Wichard. By the end of 2014, she completed her PhD. In 2015, she was promoted to Assistant Professor at Taif University. Since 2015, she has established Algal Research Laboratory and began her investigation of algal chemical ecology as well as the applications of algae in biotechnology processes such as water treatment and bioenergy production.
Abstract:
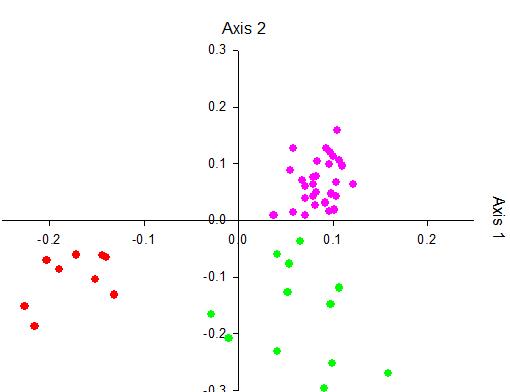
Field experiments usually give ecologically relevant results contributing to understand the ecosystem deeply. In marine research, aquacultures are one of the most popular procedures used to carry out fi eld experiments (fi gure a). In this study, the aquaculture of the green macroalga Ulva mutabilis was inoculated for the fi rst time with freshly induced gametes (7-day old germlings) to culture large volume (200 L) with small axenic gametes. Two sets of aquaculture: defi ned community (inoculated with axenic gametes of U. mutabilis and two associated bacteria: Roseovarius sp. strain MS2. and Maribacter sp. strain MS6), and undefi ned community (inoculated of U. mutabilis axenic gametes only). In defi ned community U. mutabilis showed a healthy growth and development (fi gure b) whereas in undefi ned community U. mutabilis lost its ability for growing and developing and formed only callus-like colonies (fi gure c). Multivariate statistics of the GC/MS and LC/MS analyses along with acquisition of biological metadata revealed that the waterborne metabolites in defi ned community were aff ected qualitatively and quantitatively by the growth phases of U. mutabilis as was proven in previous study of bioreactor cultures.
Yuliya E Silina
INM-Leibniz Institute for New Materials, Germany
Title: Hydrophilic interaction chromatography-tandem mass spectrometry for the cellular analysis: opportunities and cOlimpica 2enges

Biography:
Yuliya E Silina completed her Doctorate degree in Analytical Chemistry. She is a Principal Investigator in the analytical team at Leibniz Institute for New Materials, Germany, focusing on innovations in chromatography, modern mass-spectrometry and environmental sensing. She has published more than 30 papers in reputed journals.
Abstract:
Recently, a link between changes in the cellular state refl ected by nucleotide and lipid profi les has been established. The analytical determination of nucleotides is not trivial, however, because of their high polarity and hydrophilic nature. Liquid chromatography (LC) has been widely implemented for determination of nucleotides from biological samples. Most of these LC-based techniques usually require ion-pairing reagents, thus making them unsuitable for LC-mass spectrometry (MS), timeconsuming, however, and also exhibited limited chromatographic resolving and strong matrix background power for biological samples. On the other hand, because of complexity of lipids, lipids analysis is still full of challenges. Meanwhile, due to the vital roles of the changes in human physiological and pathological process, lipidomics is attracting more and more attention. Hydrophilic interaction chromatography (HILIC) has become a powerful tool for the retention of polar analytes, because of its excellent mobile phase compatibility and complementary selectivity to RP chromatography. Herein, we implemented HILIC-MS for separation and quantifi cation of low molecular weight nucleotides and phospholipids in a nanoparticle-treated lung cells and cells under at diff erent stages of hypoxia. Th e challenges, namely necessity of liquid-liquid and solid phase extraction, samples stability, dilution re-assay and matrix effect in tissues are resolved and discussed. Th e elution conditions were subsequently optimized by evaluating organic content, pH and salt concentration in the mobile phase allowing a short simple isocratic run of only 20 min for nucleotides and 9 min for phospholipids, respectively.
Publications
Silina Y E, Herbeck-Engel P and Koch M (2017) A study of enhanced ion formation from metal-semiconductor complexes in atmospheric pressure laser desorption/ionization mass spectrometry. J. Mass Spectrom. 52:43-53.
Silina Y E, Jung J, Kraegeloh A, Koch M and Fink-Straube C (2016) Interactions between DPPC as a component of lung surfactant and amorphous silica nanoparticles investigated by HILIC-ESI-MS. J. Chromatogr. B. 1030:222-229.
Silina Y E, Fink-Straube C, Hanselmann R G and Volmer D A (2016) p-Coumaric acid, a novel and effective biomarker for quantifying hypoxic stress by HILIC-ESI-MS. J. Chromatogr. B., 1020:6-13.
Peuschel H (2016) Ruckelshausen T, Kiefer S, Silina Y E and Kraegeloh A (2016) Penetration of CdSe/ZnS quantum dots into differentiated vs. undifferentiated Caco-2 cells. J. Nanobiotech. 17:70.
- High Performance Liquid Chromatography | Applications of Chromatography |Advancement in Chromatography
Location: Olimpica 2

Chair
Dusan Berek
Polymer Institute - SAS, Slovakia

Co-Chair
Mellissa Graewert
EMBL Hamburg Outstation, Germany
Session Introduction
Ebru TÜRKÖZ ACAR
Yeditepe University, Turkey
Title: Development and validation of an HPLC method for determination of the eplerenone in rat plasma
Time : 11:45-12:15

Biography:
Ebru Türköz Acar has completed her PhD at Ondokuz Mayis University, Science and Art Faculty, Turkey. She is a Researcher and Lecturer at Yeditepe University, Faculty of Pharmacy Department of Analytical Chemistry.
Abstract:
Eplerenone (EP) is an antihypertensive agent in the pharmacological group of selective aldosterone receptor antagonists (SARAs). Th e chemical structure of EP is pregn-4-ene-7,21-dicarboxylic acid, 9,11 –epoxy-17-hydroxy-3-oxo, γ-lactone methyl ester (7α, 11α, 17α). EP is slightly soluble in water and its solubility is independent from pH. Low water soluble active agents could not off er an eff ective therapy for the patients. To overcome bioavailability problems related to this property, there are some alternative ways like chemical and polymorphic modifi cations of drugs or designing appropriate pharmaceutical dosage forms. To provide an eff ective treatment, colloidal drug carrier systems of EP were prepared in this study. Investigation of loading capacity and encapsulation effi ciency of these carriers and in vitro drug release profi les, in vivo evaluation offormulations, was highly depend on a reliable quantitative analytical method. Th ere are a few methods for the quantitative determination of EP. Th ese methods mostly include expensive instruments such as LC–MS, but one exception using high performance liquid chromatography (HPLC) with UV detection was found., To assess in vivo performance of EP formulations in rats, the method was not suffi cient to provide specifi city. Th us, a new specifi c method for EP detection was needed by using the simple extraction and detection techniques. To achieve the quantifi cation of EP in the rat plasma, an HPLC method was developed and validated. EP spiked rat plasma samples were used for validation step. Then, spiked plasma was extracted and analyzed. The developed method was used to monitor the kinetic study results.
Kamal Matar
Kuwait University, Kuwait
Title: Therapeutic drug monitoring of busulfan by UPLC-tandem mass spectrometry
Time : 12:15-12:45

Biography:
Kamal Matar is an Associate Professor and Chairman of the Department of Pharmacology & Therapeutics at Kuwait University. He is also a Director of Therapeutic Drug Monitoring & Clinical Toxicology (TDM&CT) Unit, Faculty of Medicine, Kuwait University. He is involved in training Graduate students as well as Resident Physicians in the utilization of a wide variety of analytical techniques used for TDM. He completed his PhD at Cardiff University, UK, in 2000. He has published over 40 peer-reviewed articles and over 30 abstracts in international conferences. He is serving as a Reviewer for some international journals such as: Journal of Chromatography B, Journal of Pharmaceutical & Biomedical Analysis, Medical Principles & Practice, Chemotherapy, Drugs, Analytical Chemistry Insights and Journal of Antimicrobial Chemotherapy. He is a member of International Association of Therapeutic Drug Monitoring & Clinical Toxicology (IATDMCT).
Abstract:
Background: Busulfan (Bu) is an alkylating agent commonly used in preparative regimens for patients undergoing hematopoietic stem cell transplantation (HSCT) for various types of malignancies. A rapid, selective, reliable, precise, accurate, and reproducible method for quantifi cation of Bu in human plasma using Acquity UPLC system coupled to triple quadrupole tandem mass detector (TQD) has been developed and validated to be used routinely for TDM of Bu employing Busulfan-d8 as an internal standard (IS).
Methods: Th e drug and IS were extracted by ether and analyzed on Acquity UPLC BEH C18 column (2.1x50 mm, 1.7 μm). Quantitation was achieved using positive electrospray ion source (ESI+) interface employing MRM mode.
Results: Th e method was validated over the concentration range of 25–2000 ng/ml (r>0.99). Intra- and inter-run precision of Bu assay at four concentrations (50, 500, 1250 and 1750 ng/ml) ranged from 1.2 to 6.5% with accuracy (bias) varied from –10.7 to –0.2% indicating good precision and accuracy. Stability of Bu in human plasma samples at diff erent conditions showed that the drug was stable under the studied conditions.
Conclusion: The suitability of the developed method for routine TDM was demonstrated by measuring Bu in human plasma samples of patients under preparative and conditioning regimens who will undergo hematopoietic cell transplantation (HCT) for various malignancies including acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) as well as nonmalignancy conditions such as thalassemias.
Dušan Berek
Polymer Institute - SAS, Slovakia
Title: Progress in liquid chromatography of synthetic polymers
Time : 12:45-13:15

Biography:
Dusan Berek is working at Polymer Institute, Slovak Academy of Sciences in Bratislava. He served as elected member of the Presidium of the Slovak Academy of Sciences, President of the Slovak Chemical Society, Chairman of the Czecho-Slovak and Slovak National Committee of Chemistry for IUPAC. He is an author and co-author of two monographs and 250+ scientifi c papers published in refereed periodicals, proceedings and chapters of books.
Abstract:
High performance liquid chromatographic (HPLC) methods represent the most important tool for molecular characterization of synthetic polymers. Mean molar masses and molar mass distributions of linear and branched homopolymers are easily determined by size exclusion/gel permeation chromatography (SEC/GPC). As by-products, several other useful data can be assessed with SEC/GPC. Recent progress in SEC/GPC comprises improved instrumental hardware and data processing procedures. High sample throughput of the ultra-fast SEC/GPC allows acceleration of analyses, which is especially important in combinatorial material chemistry and in production control. Still, further improvements of the SEC/GPC method are needed, which include its hardware, especially columns and detectors, and also standardization of sample preparation, measurements, and data processing. SEC/GPC exhibits excellent intra-laboratory repeatability, which evokes a notion of its high reliability. Recent series of the round robin tests, however, revealed surprisingly poor inter-laboratory reproducibility of results. Evidently, accuracy of many SEC/GPC results may be rather limited. In most cases, SEC/GPC does not enable precise molecular characterization of complex polymer systems, which possess more than one distribution in their molecular characteristics. Typically, polymer mixtures, copolymers and functional polymers exhibit besides molar mass distribution also distribution in their chemical structure while e.g. stereo-regular polymer species show also molecular architecture distribution. To assess above distributions, new HPLC procedures are developed. Th ese are based on the controlled combinations of entropic (exclusion) and enthalpic (interaction) retention mechanisms within the same HPLC column or in a series of independent separation systems. Th ese approaches are denoted as “coupled polymer HPLC” and “two-, or multi-dimensional polymer HPLC”, respectively. Enthalpic retention mechanisms in HPLC of synthetic polymers include adsorption, partition, phase separation and ionic eff ects. We shall review recent progress and also problems in SEC/GPC, as well as in coupled and twodimensional polymer HPLC procedures, and outline anticipated future developments in these fields.
Publications
- Berek D (2016) Critical assessment of “critical” liquid chromatography of block copolymers J. Sep. Sci. 39:93–101.
- Clementi L A, Sišková A, Meira GR, Berek D and Vega J R (2016) Molar mass distributions in binary homopolymer blends by single step two- dimensional liquid chromatography: Operation and data treatment. Polym. Testing 52:33-40.
- Netopilík M, Janata M, Svitáková R, Trhlíková O, Berek D, Macova E, Limpouchová Z and Procházka K (2016) Chromatographic study of the conformational behavior of graft copolymers with a broad distribution of grafting densities in dilute solutions in selective solvents for grafts. J. Liq. Chromatogr. Rel. Technol. 39:50–58.
- Rollet M, Pelletier P B, Altounian A, Berek D, Maria S, Phan T N T and Gigmes D (2015) Separation of parent homopolymers from poly(ethylene oxide) and polystyrene-based block copolymers by liquid chromatography under limiting conditions of desorption – Determination of the suitable molar mass range and optimization of chromatographic conditions. J Chromatogr. A 1392:37-47.
Marco Ruijken
MsMetrix, Netherlands
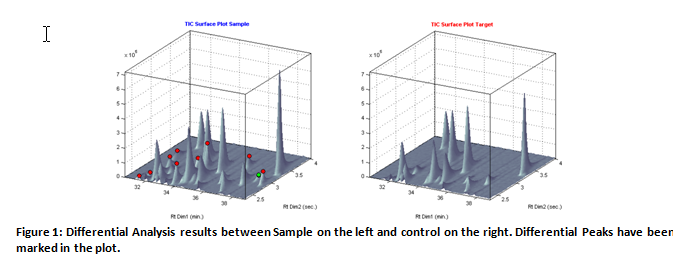
Title: All ion differential analysis in product control applications using comprehensive GCxGC/MS
Time : 14:15-14:45

Biography:
Marco Ruijken is the Owner/ Head of Research of MsMetrix, Maarssen the Netherlands. MsMetrix develops informatics solutions for LC/MS and GC/MS Data Analysis in the area of: Metabolite Profi ling, Metabolomics, Proteomics, BioMarker Discovery, and Impurity / Degradation Profi ling. Our mission is to be the premier provider of fast, affordable, user-friendly and reliable software in the above application fi elds. His educational background is in Chemometrics/Statistics and Processing of complex data. Current research topics are advanced deconvolution in GC/MS and GCxGC/MS with the focus on Differential Analysis. Furthermore, we are specialized in implementing ideas or requirements from universities or companies into our existing software tools.
Abstract:
Many applications in comprehensive GCxGC/MS relate to fi nding diff erences between a newly measured sample and a so-called reference sample. Th ese questions may typically arise in application areas like product control or during trouble shooting. Examples are: what are the new impurities present in a new batch compared to a reference batch?; why does this product behave diff erently compared to our reference batch? And; the comparison of samples in food fraud applications to detect illegally added substances. Typically for the above examples is the limited time available to solve these problems. Furthermore, most of the time only a few samples are available, which excludes the use of statistical comparison tools as applied in the fi eld of metabolomics. Although GCxGC-MS has become an invaluable laboratory analysis tool, the procedure may produce gigabytes of data per sample in four dimensions, which makes data analysis time consuming and complicated. In the presentation, new methods and soft ware tools will be presented to quickly fi nd diff erential components from a comparison between two samples only. Certainly, comprehensive GCxGC/MS is a technique having superior separation capabilities compared to 1-dimensional GC/MS, but co-elution or near co-elution still might occur, especially in complicated matrices. Whereas most soft ware tools for GCxGC/MS use processing of “TIC” data only, our new methods apply data analysis using the “all ions” approach. The implemented method allows for the detection and de-convolution of diff erential components that are not or badly separated, even in two dimensions. It will be demonstrated that processing using the “all ion” approach will substantially detect more (diff erential) components, compared to the analysis using TIC data only. Technical details of the algorithms will be explained and examples will be given from applications like food analysis, product control in flavor & fragrance industry and from base chemistry industry.
Ravi Bhushan
Indian Institute of Technology Roorkee, India
Title: HPLC enantioseparation of (RS)-Isoprenaline and enhanced detection in human plasma and commercial sample: Establishment of configuration andelution order in the absence of pure isomer
Time : 14:45-15:15

Biography:
Ravi Bhushan has an expertise in “Enantiomeric resolution of compounds of pharmaceutical importance using liquid chromatography”. So far, he has guided 28 doctoral and 50 masters’ theses, published more than 230 research papers in international refereed journals and chapters in books and encyclopedia. He edited four Special Issues of Biomedical Chromatography on chiral resolutions as Guest Editor. He received Alexander von Humboldt fellowship of Germany in 1988, and European Economic Community Fellowship in 1992. He is an elected Fellow of the Royal Society of Chemistry, London, and Fellow of National Academy of Sciences India, (FNASc). He received ‘Outstanding Teacher Award’ of the year 2007 at IIT Roorkee, and Khosla Research Prize and Silver Medal of University of Roorkee. His current research interest includes enantioseparation with both achiral phases of chromatography in the absence of any external chiral species.
Abstract:
There has been an increasing awareness that the two enantiomers of a chiral drug have diff erent pharmaceutical responses in the human body and other living systems. Th erefore, the importance of enantioseparation remains as established and challenging. (RS)-isoprenaline (Ipn) is a non-selective β-adrenergic agonist used in the treatment of bradycardia and heart block. Th e (R)-Ipn is approximately 90 times more potent than its (S)-enantiomer, therefore, determination of enantiomeric purity of this compound is essential for diagnostic purposes and therapeutic drug monitoring. With the above background and lack of literature on the work focused herein, we developed a validated analytical method for separation of enantiomers of (RS)- isoprenaline and pharmaceutical formulations. Ipn contains a reactive amino functional group (only one in close proximity to the stereogenic centers) suitable for quantitative transformation with a functionally compatible chiral reagent. Cyanuric chloride (CC, trichloro-s-triazine) was chosen for its tri-functionality and high molar absorptivity and D-phenyl glycine (D-Phg) was introduced as chiral auxiliary by substitution of one of the Cl atoms in CC. Th e second Cl atom was replaced with piperidinyl moiety as the achiral unit resulting into a structurally new CDR because such a CDR with combination of these two moieties has not yet been reported. Th e CDR was characterized. Diastereomeric derivatives of (RS)-Ipn were synthesized under microwave irradiation when the third Cl atom of CC was substituted. Th e separation of these diastereomeric derivatives was achieved using LiChrospher C18 (IxI.D. 25 cm×4.6 mm, 5 um particle size) column with mobile phase consisting of MeCN and TFA under gradient elution at fl ow rate of 1.0 mL min-1 and UV detection at 254 nm. Th e conditions for synthesis and separation were optimized by extensive experimentation with many variations at diff erent stages. Th e method was successfully applied for detection and separation of enantiomers of (RS)-Ipn in human plasma and from the commercial sample. Limit of detection values were found to be 24.6 and 26.8 ng mL-1 for the two diastereomeric derivatives. Geometry optimized lowest energy structures of diastereomeric derivatives of (RS)-Ipn were developed using a standard Gaussian soft ware program which helped in establishing the confi guration and elution order of the diastereomeric derivatives. Th e method can be used not only for control of enantiomeric purity of Ipn in industrial/commercial samples but also for enantioseparation and determination of other structurally similar pharmaceutically important molecules for routine analysis as well.
Katsuhiro Maeda
Kanazawa University, Japan
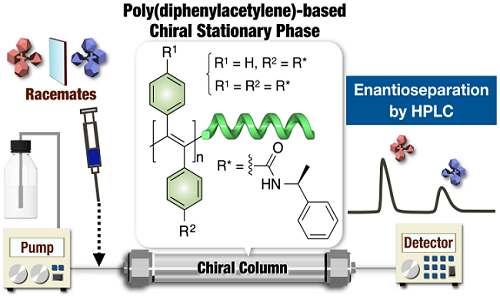
Title: Enantioseparation on optically active poly(diphenylacetylene)s as chiral stationary phases for HPLC
Time : 15:15-15:45

Biography:
Katsuhiro Maeda completed his BS in 1993, MS in 1995, and PhD in 1998 at Nagoya University. In 1998, he joined the Graduate School of Molecular Design and Engineering at Nagoya University as an Assistant Professor and was promoted to Associate Professor in 2002. He moved to Kanazawa University in 2008 and was appointed as a full Professor in 2015. He has published more than 80 original papers.
Abstract:
Enantioseparation by high performance liquid chromatography (HPLC) is recognized as one of the most popular and eff ective methods for both analyzing the composition of enantiomeric mixtures and obtaining pure enantiomers, and a large number of chiral stationary phases (CSPs) have been developed to resolve various racemates. Several optically active helical poly(phenylacetylene)s bearing polar functional groups as a chiral recognition site have been reported to exhibit good chiral recognition abilities toward some racemates due to the preferred-handed helical conformation when used as CSPs for HPLC. On the other hand, the number of optically active poly(diphenylacetylene)s bearing polar functional groups reported so far is very limited and poly(diphenylacetylene)-based CSPs have not yet been reported. In this study, we synthesized optically active poly(diphenylacetylene) derivatives bearing amide groups as eff ective chiral recognition sites by the macromolecular reaction of the optically inactive precursor poly(diphenylacetylene)s bearing carboxy groups with optically active amines and investigated their chiral recognition abilities as CSPs for HPLC. The obtained polymers showed good chiral recognition ability towards diverse racemates when used as CSPs for HPLC. Notably, the preferred-handed helical conformation was induced on the polymer backbone by the thermal annealing process, which was applied aft er the introduction of the optically active pendants via a polymer reaction. Th e chiral recognition abilities of the polymers with a preferred-handed helicity were greater than those of the as-prepared polymers without a preferred-handed helicity. These results indicated that the macromolecular helicity induced in the polymer backbone by thermal annealing as a consequence of the eff ect of the chiral pendant groups was playing an important role in the high chiral recognition ability of these poly(diphenylacetylene) derivatives.
Publications
- Maeda K, Maruta M, Sakai Y, Ikai T and Kanoh S (2016) Synthesis of optically active poly(diphenylacetylene)s using polymer reactions and an evaluation of their chiral recognition abilities as chiral stationary phases for HPLC. Molecules 21:1487-1500.
- Maeda K, Maruta M, Shimomura K, Ikai T and Kanoh S (2016) Chiral recognition ability of an optically active poly(diphenylacetylene) as a chiral stationary phase for HPLC. Chem. Lett. 45:1063-1065.
- Yashima E, Ousaka N, Taura D, Shimomura K, Ikai T and Maeda K (2016) Supramolecular helical systems: Helical assemblies of small molecules, foldamers, and polymers with chiral amplification and their functions. Chem. Rev. 116:13752-13990.
- Maeda K, Miyagawa T, Furuko A, Onouchi H and Yashima E (2015) Dual memory of enantiomeric helices in poly(phenylacetylene)s induced by a single enantiomer through helix inversion and dual storage of the enantiomeric helicity memories. Macromolecules 48:4281-4293.
- Shimomura K, Ikai T, Kanoh S, Yashima E and Maeda K (2014) Switchable enantioseparation based on macromolecular memory of a helical poly-acetylene in the solid state. Nat. Chem. 6:429-434.
